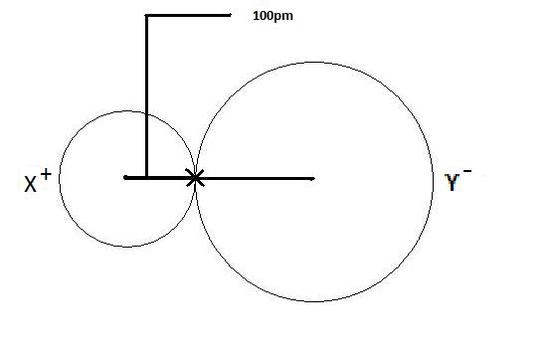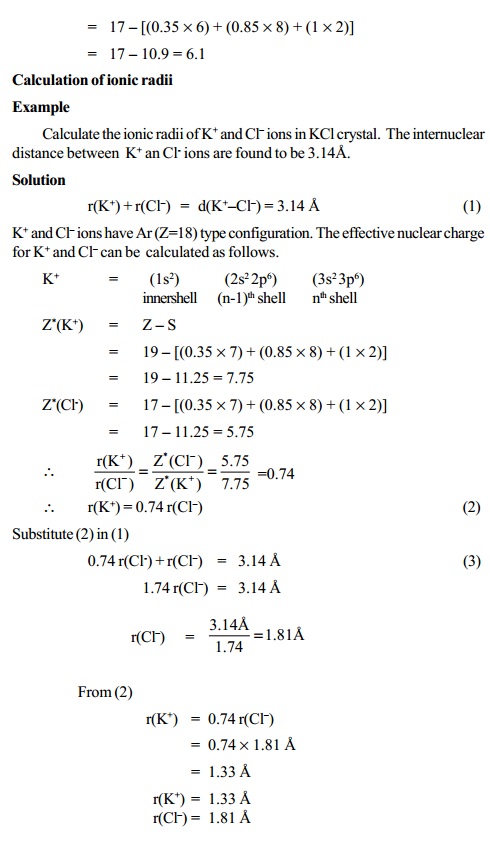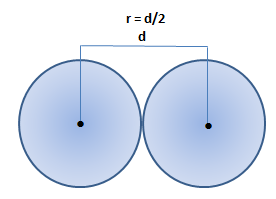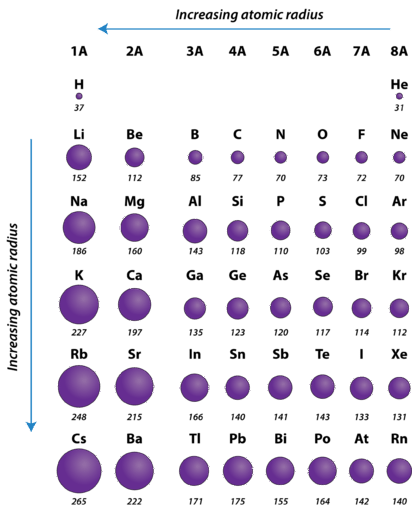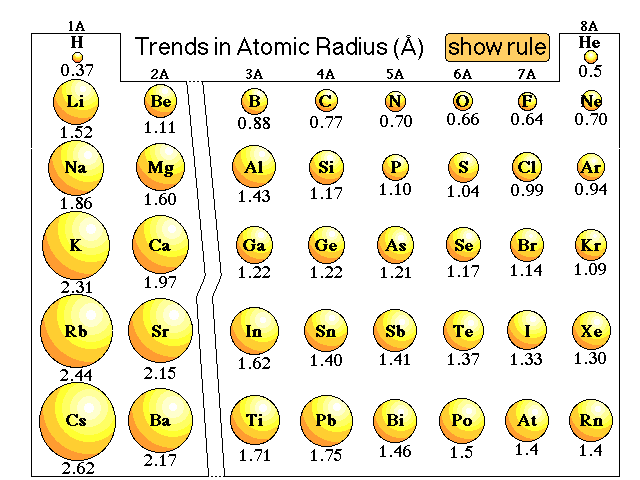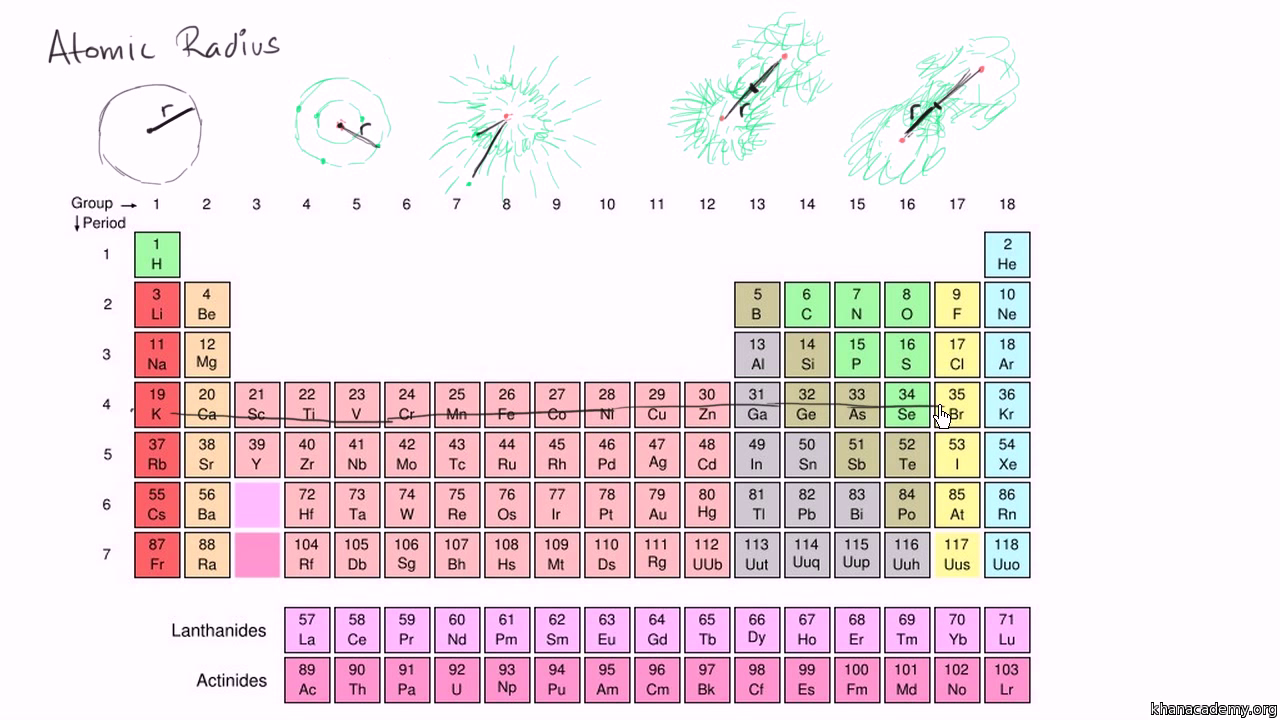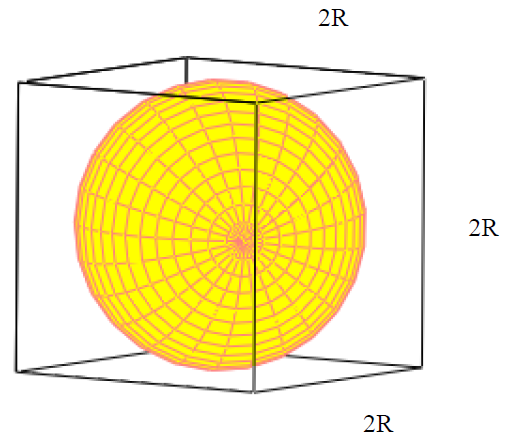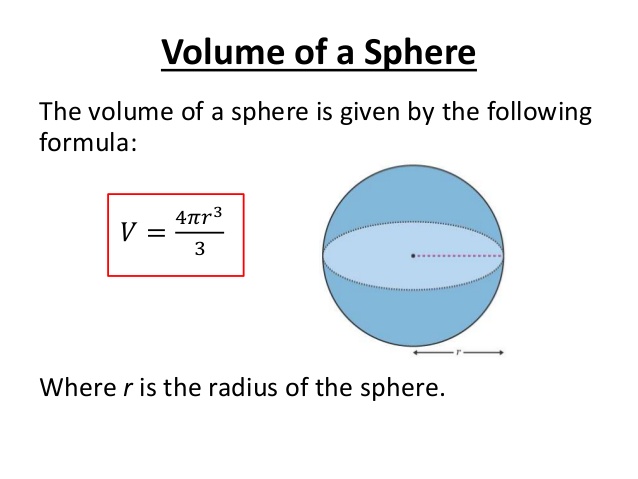
Atomic radius is of order 10^-8 cm and nuclear radius is of order 10^-13. calculate what fraction of atom is occupied by nucleus? | Socratic

Lithium metal crystallizes in a body - centered cubic crystal. If the length of the side of the unit cell of lithium is 351 pm, the atomic radius of lithium will be:
What is the formula to calculate the radius of an orbit of the atom and velocity of the specific shell of the atom.
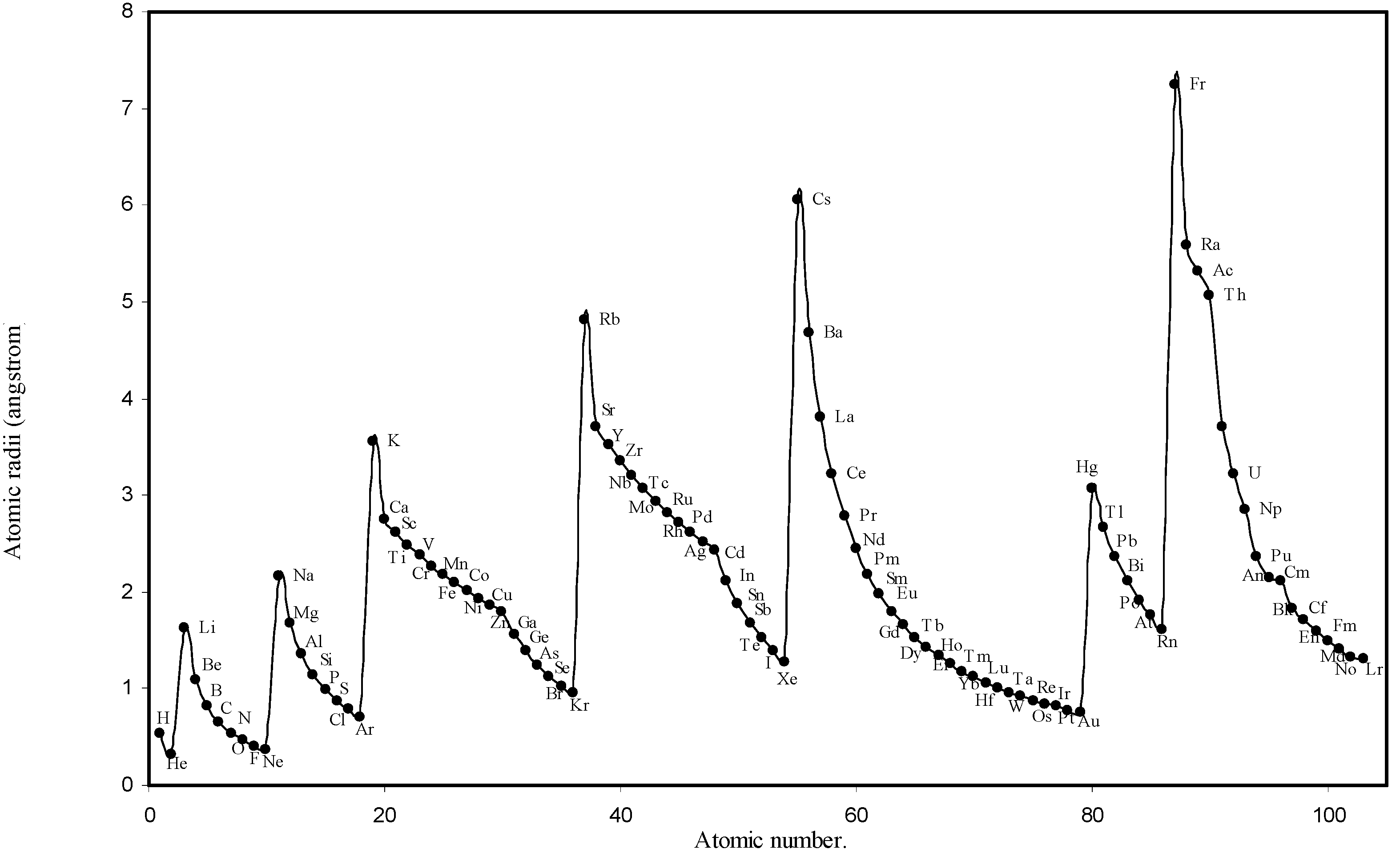
IJMS | Free Full-Text | Theoretical Calculation of Absolute Radii of Atoms and Ions. Part 1. The Atomic Radii
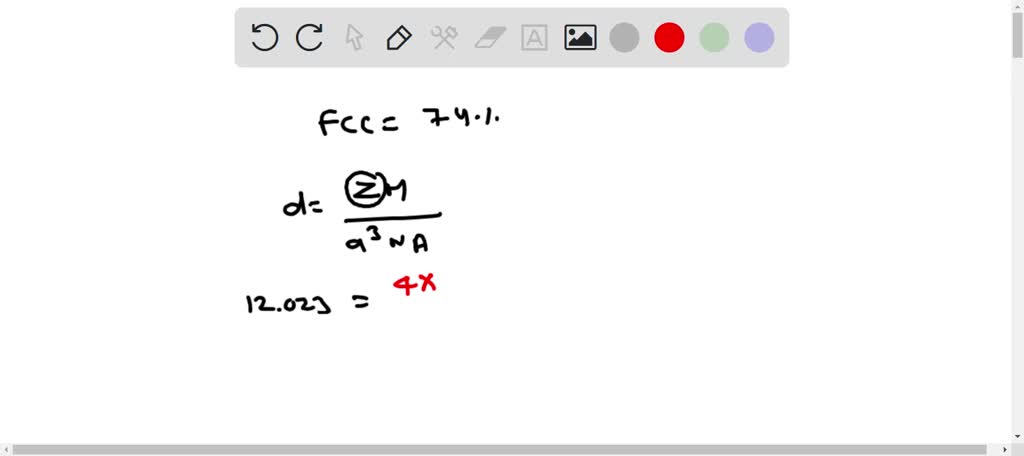
SOLVED: Palladium (at. wt. = 106) crystallizes in a face-centered cubic unit cell. Its density is 12.023 g/cm3 . Calculate the atomic radius of palladium and its packing efficiency.