PDF) Calculation of excess enthalpy of binary mixtures with using of excess volume experimental data

SOLVED: Calculate the mass of water produced when 9.47 8 of u methane CHa reacts with an excess of oxygen in the following reaction: CH4 + 202 COz + 2Hz0 HzO molar

Calculating the Heat of Reaction from Molar Reaction Enthalpy and the Mass of a Reactant | Chemistry | Study.com

SOLVED: The molar excess Gibbs free energy of formation of solid solutions in the 'system Au-Ni can be represented by G*s '=XiXAu(QAIAOX Au + 38280X Ni 14230XAuX Ni 2660 Calculate the activities
27. A metal chloride dissolve endothermic , if 7.45 g of its anhydrous form dissolve in excess of of water the amount of heat absorbed is X KJ. Calculate enthalpy of solution
Determination of the excess thermodynamic functions of components of binary salt melts in infinitely diluted solution on the bas
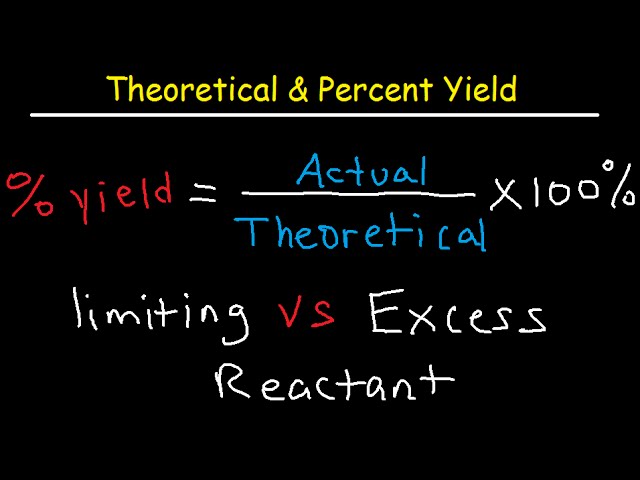
Theoretical, Actual, Percent Yield & Error - Limiting Reagent and Excess Reactant That Remains - YouTube

When 7.45 g of metal chloride dissolved in excess of water, the amount of heat absorbed is X kJ. Calculate the enthalpy of solution if molar mass of chloride is 74.5.